Topical Tretinoin 0.05 % Cream May Help Some with Limited Alopecia Areata
Alopecia areata is an autoimmune disease that affects 2 % of patients. There are well over 2 dozen treatments available
More than Shots: The 30 Treatment Options for Alopecia Areata
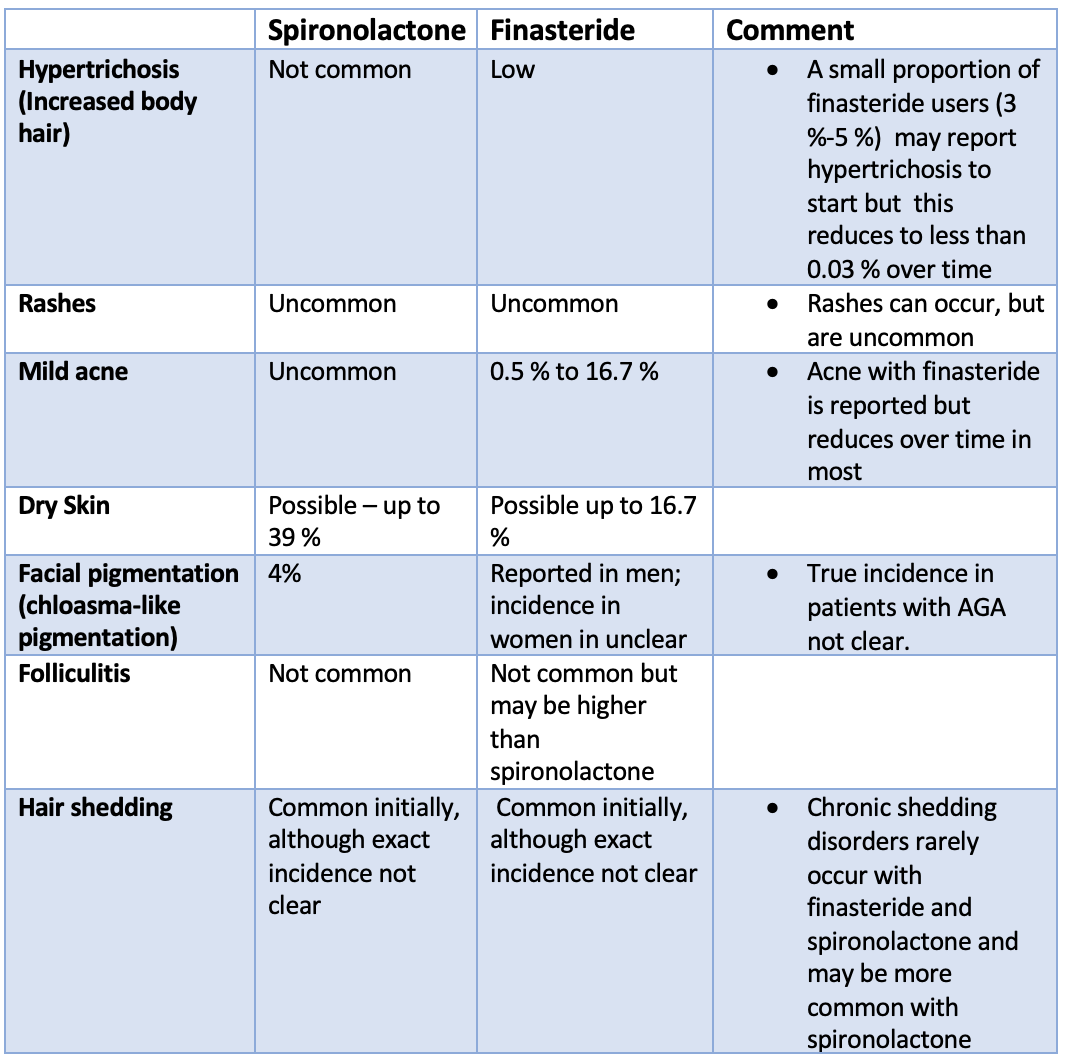
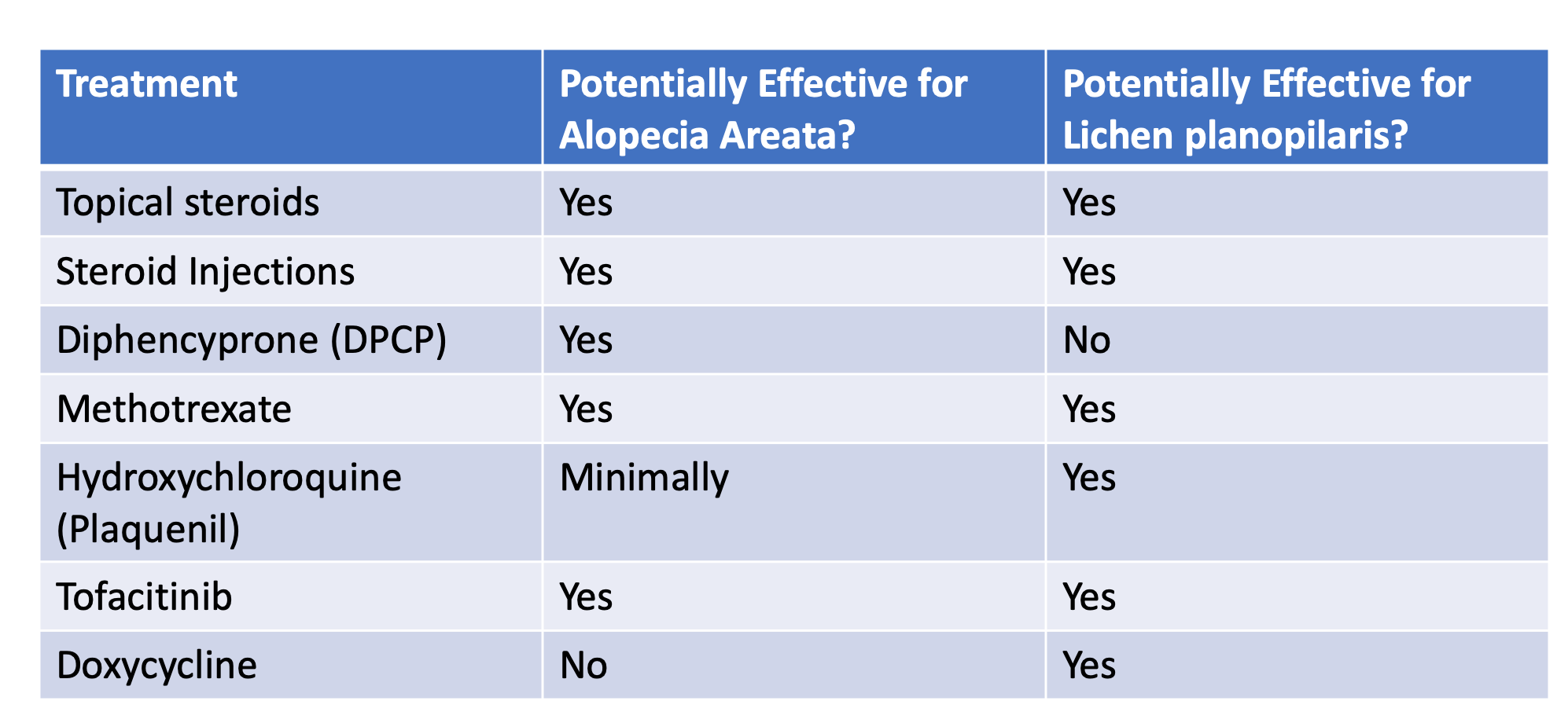
Topical options are often viewed as safer than systemic options (pills) because the body gets exposed to less medication. Topical treatment options include: Topical steroids, Topical bimatoprost, Essential oils, Anthralin, Squaric acid, Diphencyprone, Minoxidil, Topical tofacitinib, Topical ruxolitinib, Onion juice, Garlic gels, Topical capsaicin and Topical retinoids
Treatments for alopecia are generally of three main types
1) those treatments that reduce inflammation around the hairs so the hairs can grow. Examples include topical steroids and topical tofacitinib.
2) those treatments that simply stimulate hair growth so that hairs can push through the skin and keep growing despite their inflammation. These options may also reduce inflammation as well. Examples include minoxidil and low level laser.
3) those treatments that cause inflammation somewhere else such as the surface of the skin layer so that inflammation gets slowly reduced from around the follicles. A variety of treatments fall into the third category include anthralin, Diphencyprone, squaric acid and tretinoin.
Use of Tretinoin in Treating AA
Today we’ll focus on the use of tretinoin in treating alopecia areata.
Tretinoin is a type of vitamin A. It is used as an acne treatment and as an anti-aging treatment and has been available to patients since the early 1970s. Several studies to date have suggested that tretinoin may have some benefit in the treatment of alopecia areata. It is not effective for everyone and is likely less effective than standard treatments like topical steroids, steroid injections and the oral immunosuppressants. But a small handful of studies suggest that it’s an option to be considered.
STUDY 1: Das and colleagues, 2010
In 2010, Das and colleagues published a study of 80 patients that sought to compare the benefits of 3 treatments, namely a strong topical steroid known as betamethasone diproprionate, tretinoin 0.05 % and anthralin paste 0.25% in the treatment of limited alopecia areata. A placebo group was also included in the study bringing the total number of study groups to four. Treatments were applied twice daily. Patients with alopecia areata in this study had more limited disease and could only be included in the study in their patches were less than 5 cm in diameter and if they had less than 5 patches in total. Results of the study showed that 70 % of patients received topical steroids had an improvement compared to 55 % with tretinoin, 35 % with anthralin and 20 % with placebo.
STUDY 2: Hussein 2020
In 2020, Hussein performed a study comparing the benefits of betamethsone diproprionate topical steroid to tretinoin 0.05% in 50 patients with limited alopecia areata. Treatments were applied twice daily. Similar to the 2010 Das study, patients could only be included in the study if they had less than 5 patches and if they had less than 25 % scalp involvement. After 12 weeks, 72 % of patients receiving the topical steroid had statistically significant clinical improvement compared to 36 % receiving tretinoin 0.05%.
STUDY 3: Kubeyinje and Mathur, 1997
A 1997 study showed that use of tretinoin in patients receiving steroid injections could have added benefit. The authors of the study evaluated the efficacy and safety of 0.05% tretinoin cream as an adjunctive therapy or ‘add on’ treatment with intralesional triamcinolone acetonide in aiopecia areata, by comparing the result of treatment with monthly intralesional triamcinolone acetone and daily application of 0.05% tretinoin cream in 28 patients with alopecia areata with 30 similar patients treated with only monthly intralesional triamcinolone acetonide as controls. Results at 4 months showed more than 90% regrowth in 85.7% of patients on triamcinolone acetonide and tretinoin cream, as compared with 66.7% of patients receiving only triamcinoione acetonide.
STUDY 4: Much, 1976
A 1976 study was among the first published studies to show benefits of tretinoin in treating alopecia areata.
Conclusion and Summary Points
With specific treatments like JAK inhibitors and others, the future of alopecia areata is bright. However, it is critically essential that we not forget our past and where we have come from over many decades of study. Physicians treating alopecia areata must appreciate that role of very simple and relatively inexpensive treatments and the large number of patients with limited alopecia areata they may potentially help. Tretinoin is on that list of simple treatments. Tretinoin is a topical treatment that certainly does not help everyone but may have a role in patients with more limited disease. In patients of mine with a few patches who can not tolerate minoxidil or who can not tolerate steroids, tretinoin remains an option.
We use tretinoin with several treatment including 1) tretinoin with topical minoxidil, 2) tretinoin with topical steroids, 3) tretinoin with steroid injections and 4) tretinoin with diphenyprone or squaric acid.
Side effects including redness and irritation and that is in fact the reason that typically use it in alopecia areata. In other words, it is a side effect but not a concerning one as that is in fact that desired effect. We ask patients to keep close follow up with our office so that we can assist them in finding the right dose that works for them. Some need use daily, some twice weekly and some just 2-3 times per week. Tretinoin must not be used during pregnancy.
When used alone, I may prescribe tretinoin daily to start and then increase to twice daily. When used in conjunction with other treatments, we often start tretinoin 2-3 times weekly for a few weeks and then increase to 4 times weekly and then five times weekly and then six times weekly and finally using it daily.
REFERENCE
Baird KA. Alopecia areata. Arch Dermatol. 1971;104:562-3.
Das et al. COMPARATIVE ASSESSMENT OF TOPICAL STEROIDS, TOPICAL TRETENOIN (0.05%) AND DITHRANOL PASTE IN ALOPECIA AREATA. Indian J Dermatol. 2010 Apr-Jun; 55(2): 148–149.
Hussein AA. A comparative study of the outcomes of potent topical steroids versus topical tretinoin in patchy alopecia areata of scalp. Int J Res Dermatol. 2020 Jan;6(1):111-114
Kubeyinje EP, C'Mathur M. Topical Tretinoin as an Adjunctive Therapy With Intralesional Triamcinolone Acetonide for Alopecia Areata. Clinical Experience in Northern Saudi Arabia. Int J DermatolJ Dermatol. 1997 Apr;36(4):320
Much T. Treatment of alopecia areata with vitamin A acid. Z Hautkr, 51 (1976), pp. 993-998
.