What are the side effects of Low Dose Naltrexone (LDN)?
Low dose naltrexone: What side effects are possible?
Low dose naltrexone is increasingly studied in the field of hair loss, mainly in the areas of scarring alopecia and alopecia areata. To date, there remains only limited evidence that these drugs have a role. We continue to study them in our clinic.
Side effects: What side effects are possible?
Dr. Donovan generally counsels patients about the top side effects including
1. Difficultly sleeping.
This is usually just for the first week. If trouble sleeping go beyond this, one can reduce the dose to 3 mg or 1.5 mg
2. More vivid dreams.
This is seen in approximately 37 % of LDN users and can decrease over time.
3. Reduced need for thyroid medication.
Patients with autoimmune thyroid disease who take thyroid medications may want to start with a 1.5 mg dose and monitor their TSH every 2-4 weeks. This is to prevent a change from a hypothyroid/euthyroid state to a hyperthyroid state. Many patients with LDN require less thyroid supplementation while on LDN.
4. Headaches.
Headaches have been noted to be increased compared to placebo.
5. Anxiety (rare).
To date, there is not a consistent increase in headaches in the frequency of anxiety in LDN users compared to placebo.
6. Tachycardia (increased heart rate)
Although tachycardia is something we watch for, to date, there is not a consistent increase in headaches in the frequency of abnormal heart rhythms in LDN users compared to placebo in studies conducted to date.
7. Rare - Fatigue, Loss of appetite, nausea, mood swings, mild disorientation
Side effects in the clinical studies
There are only a limited number of well conducted studies examining side effects of low dose naltrexone. By well conducted studies, one is specifically referring to studies that compared side effects of LDN to placebo. Here are some studies that guide our understanding of side effects of LDN
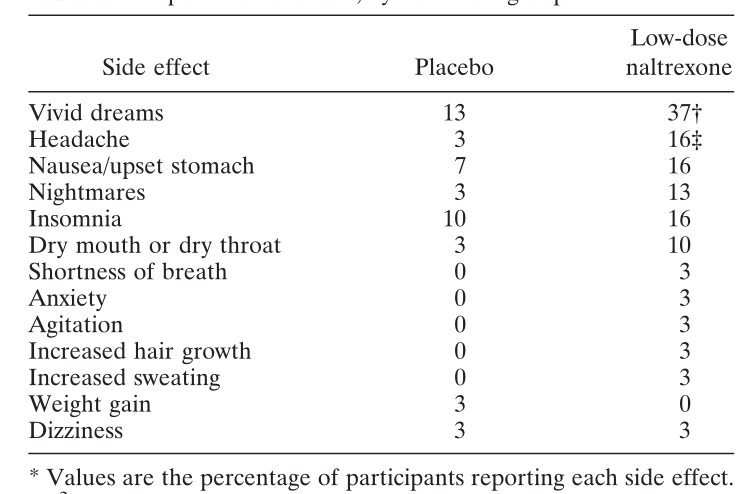
1. Younger and colleagues, 2013.
A study of 31 patients with fibromyalgia by Younger and colleagues showed that headaches and vivid dreams were by far the most important of the side effects of LDN compared to those using placebo. Other side effects did not appear statistically different in LDN users vs placebo (at least based on the small numbers).
SOURCE: Younger and colleagues. Arthritis Rheumatism 2013.
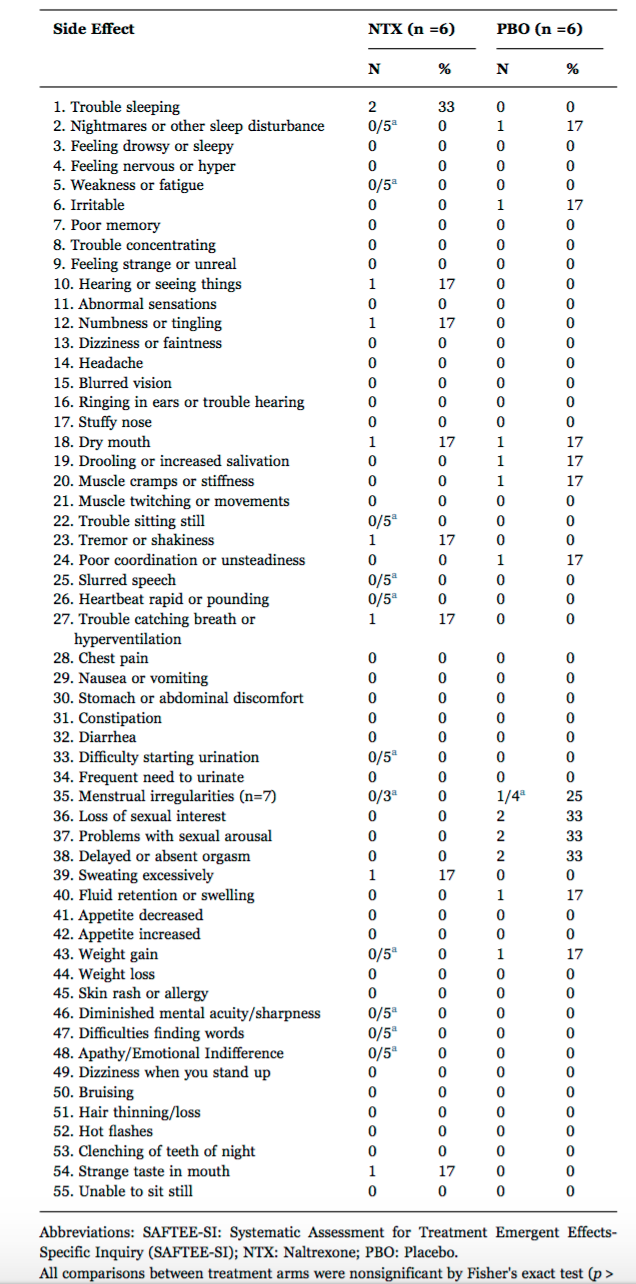
2. Mischoulon et al, 2017
A randomized trial of 1 mg twice daily low dose naltrexone (LDN) was studied by Mischoulon and colleagues in 12 patients with depression. Their study was small but differences in side effects between the treatment (NTX) and placebo (PBO) groups were not appreciable.
Mischoulon D, et al. J Affect Disord. 2017.
3. Laser Sharafaddinzadeh et al, 2010
In a randomized study study of 106 patients with multiple sclerosis, the main side effects were nausea, epigastric pain, mood alteration, mild irritability, headache, and joint pain.
4. Mohammad Ali Seifrabiei et al, 2008
A randomized study of low dose naltrexone in 89 hematologic patients showed that LDN was associated with better appetite, reduced nausea and vomiting compared to users of placebo. There were no differences in insomnia in this study.
Mohammad Ali Seifrabiei et al. Am J Applied Sciences 2008
5. Smith et al, 2013
Small studies in children receiving low dose naltrexone for inflammation bowel disease (Crohn's disease) showed no differences in sleep, dreams, twitching, headaches, appetite, nausea, or double vision.
References
Younger J, et al. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Randomized controlled trial. Arthritis Rheum. 2013.
Mischoulon D, et al. Randomized, proof-of-concept trial of low dose naltrexone for patients with breakthrough symptoms of major depressive disorder on antidepressants. Randomized controlled trial. J Affect Disord. 2017.
Smith JP, et al. Safety and tolerability of low-dose naltrexone therapy in children with moderate to severe Crohn's disease: a pilot study. Randomized controlled trial. J Clin Gastroenterol. 2013.
David Mischoulon et al. Randomized, proof-of-concept trial of low dose naltrexone for patients with breakthrough symptoms of major depressive disorder on antidepressants. Journal of Affective Disorders 2013.
Laser Sharafaddinzadeh et al. The effect of low-dose naltrexone on quality of life of patients with multiple sclerosis: a randomized placebo-controlled trial. Multiple sclerosis 2010.
This article was written by Dr. Jeff Donovan, a Canadian and US board certified dermatologist specializing exclusively in hair loss.